
- #Reactivity of carboxylic acid derivatives mcat how to
- #Reactivity of carboxylic acid derivatives mcat full
In the second step, the C-O pi bond is re-formed, resulting in breakage of the C-O sigma bond. In the first step, a hydride from aluminum forms a new C-H bond, breaking the C-O pi bond. ( See article – Nucleophilic Acyl Substitution) We’ve seen that carboxylates will not undergo addition with most nucleophiles. In the presence of most nucleophiles, formation of a carboxylate signals the end of the reaction. That’s one key reason why NaBH 4 is typically used for simple reductions – at cold temperatures, it reacts slowly and controllably with alcoholic solvents, unlike LiAlH 4. For these reasons extreme caution is used when handling LiAlH 4 and it is never left out on the bench for any extended period, as it will react with water vapor from the air.
#Reactivity of carboxylic acid derivatives mcat how to
( See article: How to Use a pK a Table).Īcid-base reactions of LiAlH 4 tend to be violently exothermic, and generate (flammable) hydrogen gas, besides. Since acid-base reactions are favored when a stronger acid will be converted to a weaker acid, this will rapidly generate the carboxylate salt (the conjugate base of the carboxylic acid) and hydrogen gas. Recall that the pK a of H 2, the conjugate acid of hydride (H-) is about 36 whereas the pK a of the carboxylic acid is around 4. What might be the very first reaction to happen here? Lithium aluminum hydride is strongly basic. So how does the reaction of LiAlH 4 with carboxylic acids work? Reduction of Carboxylic Acids By LiAlH 4 – The Mechanism One key difference is in the reduction of estersto primary alcohols, which NaBH 4 does only slowly (if at all).Ī detailed, reproducible step-by-step procedure from Organic Syntheses can be found hereĪ second reaction that LiAlH 4 will perform that NaBH 4 will not is the reduction of carboxylic acids to primary alcohols.Īs well as reductions of nitriles, amides, epoxides, and alkyl halides (and more, which we won’t cover)ģ. LiAlH 4 will also perform reductions that NaBH 4 is unable to do, or at the very least, do them much more quickly.

Walter Szarek was fond of saying, “Using LiAlH 4 for this reaction is like using a sledgehammer to kill a fly!”). LiAlH 4 will reduce aldehydes and ketones just like NaBH 4 .įor practical reasons NaBH 4 is much more convenient to use for these reactions and there is no advantage to using LiAlH 4 unless you also plan on reducing every other functional group in sight. Lithium borohydride (LiBH 4) is more reactive than NaBH 4. (There is a bit more to it than what this answer suggests.
#Reactivity of carboxylic acid derivatives mcat full
What do you think the full Lewis structure of LiAlH 4 looks like? Short version – a reducing agent forms C-H bonds while breaking C-O bonds) ( See article – Oxidation and Reduction in Organic Chemistry.
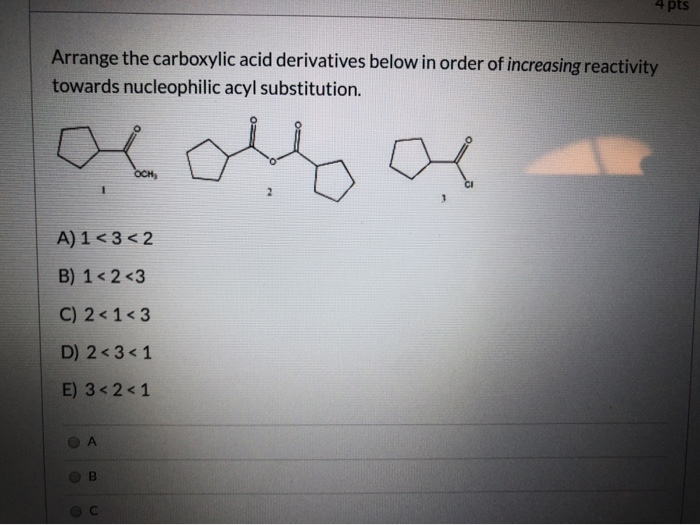
If you’re not familiar with what is meant by “ reducing agent” in organic chemistry, a refresher can be found here. Lithium aluminum hydride (LiAlH 4) is a strong reducing agent with a particular utility for carboxylic acid derivatives.

Lithium Aluminum Hydride (LAH, LiAlH 4) For the Reduction of Carboxylic Acid Derivatives


 0 kommentar(er)
0 kommentar(er)
